ISO and IEC Standards for SaMD: Breakdown of medical devices
 8 October 2024
8 October 2024Software as a medical device (SaMD) plays a critical role in healthcare. Its precision and reliability are vital. The medical device industry is quickly adapting to new software advancements. The International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC) set essential medical device software standards. Compliance with these standards is a must for entering the market.

Adopting ISO and IEC standards boosts patient safety and enhances performance. These standards shape digital health tools. The FDA places great importance on these guidelines. Together, ISO 13485:2016 and ISO 14971:2019 highlight a commitment to quality and risk management.
Software holds a special place among medical devices today. Following specific standards lets SaMD startups like Sierra QMS improve their product development. They face fewer obstacles during FDA audits. Recently, more manufacturers focus solely on software. This approach moves away from traditional hardware methods. SaMD is becoming crucial in patient care, showing its increased relevance.
Key Takeaways
- ISO 13485:2016 and ISO 14971:2019 are key for quality and risk management in SaMD development.
- IEC standards such as IEC 62304, IEC 62366, and IEC 60601-1:2 address software life cycles and usability within medical devices.
- Compliance with ISO and IEC standards aids in smooth regulatory approval and faster market access.
- SaMD’s quality and effectiveness are heavily scrutinized by the FDA and other global regulatory entities.
- The shift toward purely software-based medical solutions amplifies the importance of understanding ISO and IEC regulations.
- Clarity and compliance in SaMD development are critical factors in the FDA’s updated guidelines and premarket submissions.
Understanding Software as a Medical Device (SaMD)
The medical device software world is always changing. Software as a medical device (SaMD) is changing healthcare in big ways. SaMD is software that has medical uses and works on its own, without needing physical device hardware. It can do things like diagnose diseases, help manage patient health, or guide surgeries. It’s important because it works by itself to improve how we treat patients.
SaMD is different from traditional medical devices. It works without needing any physical devices. Governments around the world have set rules for SaMD because it can make treatment better. It can make healthcare more available, more personal, and let doctors watch health in real time. Making sure SaMD meets IEC 82304-1 standards is key. This makes sure it’s safe for the market and for people to use.
Classification of SaMD
How we classify SaMD depends on its use, risks, and how complex it is. Groups like the FDA in the U.S., Health Canada, and the European Medicines Agency put them in risk categories. There are three main risk levels depending on how much risk they have:
| Risk Class | Focus | Examples |
|---|---|---|
| Class I | Low Risk | Healthcare management apps |
| Class II | Moderate Risk | Diagnostic support software |
| Class III | High Risk | Software that guides surgical procedures |
Overview of Key ISO and IEC Standards for Medical Devices
In the medical device world, especially Software as a Medical Device (SaMD), meeting the right ISO and IEC standards is key. These standards are important for making sure medical devices are safe, work well, and meet legal rules. They deal with everything from quality and risk management to how software should be made and kept up.

ISO 13485: Quality Management Systems
The 2016 version of ISO 13485 talks about quality management system requirements for the medical device industry. It’s vital for making sure devices are made well from start to finish. The standard also helps keep an eye on devices after they’re sold, which is important for keeping up quality.
ISO 14971: Risk Management for Medical Devices
ISO 14971, updated in 2019, is a key standard for managing risks in medical devices. The FDA supports it. It gives a way for makers to find, assess, control, and keep track of risks during the device’s whole life. This is crucial for keeping devices safe and working right.
IEC 62304: Medical Device Software Lifecycle Processes
The IEC 62304 standard guides on software life cycle for medical devices. It stresses the importance of having a clear process for developing software. Following this standard helps ensure software for medical devices is safe and does its job while being made in a controlled way.
IEC 82304-1: Health Software – General Requirements
IEC 82304-1 sets the general rules for health software, going beyond just SaMD. It talks about making software usable, safe, and easy to maintain. This provides a full guide for developers to make their products meet the highest safety and effectiveness standards.
| Standard | Focus Area | Relevance to SaMD |
|---|---|---|
| ISO 13485:2016 | Quality Management Systems | Essential for consistent production quality and regulatory compliance |
| ISO 14971:2019 | Risk Management | Key to identifying and controlling risks throughout the device lifecycle |
| IEC 62304:2006 | Software Life Cycle Processes | Guides safe and effective software development within regulatory framework |
| IEC 82304-1 | General Health Software Requirements | Addresses broader health software issues beyond the scope of SaMD |
Leverage our expertise to align your SaMD with ISO and IEC standards, securing your medical device’s market readiness.
Compliance and Regulatory Requirements for SaMD
Ensuring that software as a medical device (SaMD) complies with laws is key to its success. This means deeply knowing what the FDA and other global agencies require. With SaMD growing fast, staying up-to-date with international rules is vital.
Role of FDA and Other Regulatoryves
The FDA is crucial in shaping the rules for SaMD in the U.S. It is also a key player in the International Medical Device Regulators Forum (IMDRF). Thus, the FDA’s policies often influence regulations worldwide. Agencies everywhere work to make sure SaMD is safe and effective, following international standards like ISO 13485 and IEC 62304.
Compliance Pathways for SaMD
The FDA provides clear compliance guidelines for SaMD. These include adhering to 21 CFR Part 820 and ISO 14971, focusing on quality and risk management. Aligning with these standards shows the FDA, and other bodies, that patient safety is a priority. Companies should talk early to the FDA to understand these complex requirements better.

Another key to compliance is following software lifecycle guidelines from ISO 62304 and data privacy rules from the GDPR for SaMD. Developers must analyze risks from the start to tackle any safety or compliance issues head on.
- Starting compliance checks early helps companies handle regulatory challenges.
- Ensuring user safety by closely following standards and adding necessary features like alarms is crucial.
- Using a specialized quality management system, like Greenlight Guru’s MedTech QMS, eases following these complex rules.
Risk Management in SaMD Development
In the world of SaMD (software as a Medical Device), managing risks well is very important. Using ISO 14971 in the software creation process helps find, study, and manage possible dangers. This matches SaMD development with the main standards of regulation.
Implementing ISO 14971 in Software Development
ISO 14971:2019 sets out clear guidance for managing risks in the medical software field. By adopting ISO 14971, developers make sure that finding, evaluating, and managing risks is at the heart of what they do. This is key as the EU’s Medical Device Regulation (MDR 2017/745) and the U.S.’s rules like 21 CFR Part 820 have strict risk management needs for medical software. A well-planned risk management process, as ISO 14971 outlines, can lower hazards. This makes the software safer for users and works better.
Hazard Analysis and Risk Control
The process of hazard analysis starts with spotting potential harm sources, deciding how risky they are, and then managing those risks. It’s vital for the teams making software to think about risks through the entire product’s life. This includes the design, development, and after it’s sold. For SaMD, it’s important to consider special risks from how the software is used and how people interact with it. Important points like usability (talked about in the EU MDR’s Chapter 1 Section 22) and stability (mentioned in Annex 1) are key parts of managing risks well.
Keeping up with updates after the product is out by doing things like customer surveys and CAPA (Corrective and Preventative Actions) is critical. ISO 14971:2019’s Clause 4 says it’s essential to have a risk management system that can change with new data. This makes sure the SaMD stays safe and works well over time.
Using these risk management practices in making medical software meets regulatory rules. It also speeds up getting important medical tech to market. A strong focus on finding and managing hazards is crucial for SaMD solutions to be successful and reliable.
Quality Management in SaMD
Quality management keeps Software as a Medical Device (SaMD) up to standard. It’s vital for creating a Quality Management System (QMS) that fits medical software. This helps make sure products are safe and meet regulations. It also makes medical software more effective and follows ISO 13485’s strict rules.
Applying ISO 13485 to Software Projects
ISO 13485 is the best guide for quality management in making medical devices, including software. It gives a plan for making software projects stick to quality processes. ISO 13485 improves important areas like risk handling, keeping track of documents, and meeting regulations.
This helps make medical software safe and reliable.
Ensuring Quality and Safety Through Life Cycle
The medical software life cycle gains from strict quality management. This approach is key from the start to after the product is sold. It not only meets rules but also makes sure the product works well and is safe.
Life cycle stages like design, making, and keeping up need constant attention. This is to stay up to date with safety needs and industry standards.
ISO 13485’s role in the medical software industry is shown through stats from the International Medical Device Regulators Forum (IMDRF). These stats also show how it’s used in the industry today:
| Principle | Implementation |
|---|---|
| Leadership and Organizational Support | 86% of workers with compliance jobs feel strong leadership support. This helps build a culture focused on quality and compliance. |
| Life Cycle Support Processes | Detailed strategies from planning to putting into action are key. They help expand QMS across organizations of different sizes. |
| Realization and Use Processes | Important steps include managing requirements, designing, developing, and maintaining. These are crucial for quality and safety in medical software. |
Software Development Process for Medical Devices
The software development process for medical devices must be very careful. It has to meet tough requirements for different stages like planning, design, validation, and after launch activities. This thorough method makes sure medical devices work well and follow strict rules from regulatory organizations.
Planning and Requirements Definition
The first step is making a detailed plan. This plan figures out what the device needs to do and what the users need from it. It must match up with standards from the IEC 62304 and FDA rules.
Software Design and Architecture
The creation of the software’s design and structure is a major part. It involves making detailed plans that make the device efficient and reliable. By using top-notch architectural solutions, makers can create software that grows and still meets medical and tech standards.
Software Validation and Verification
Checking that the software does its job right is key. This step makes sure it meets the set performance and safety standards. It’s a crucial part for catching and fixing risks before the device goes to market. This way, it gains the trust of users and stakeholders.
Maintenance and Post-Market Surveillance
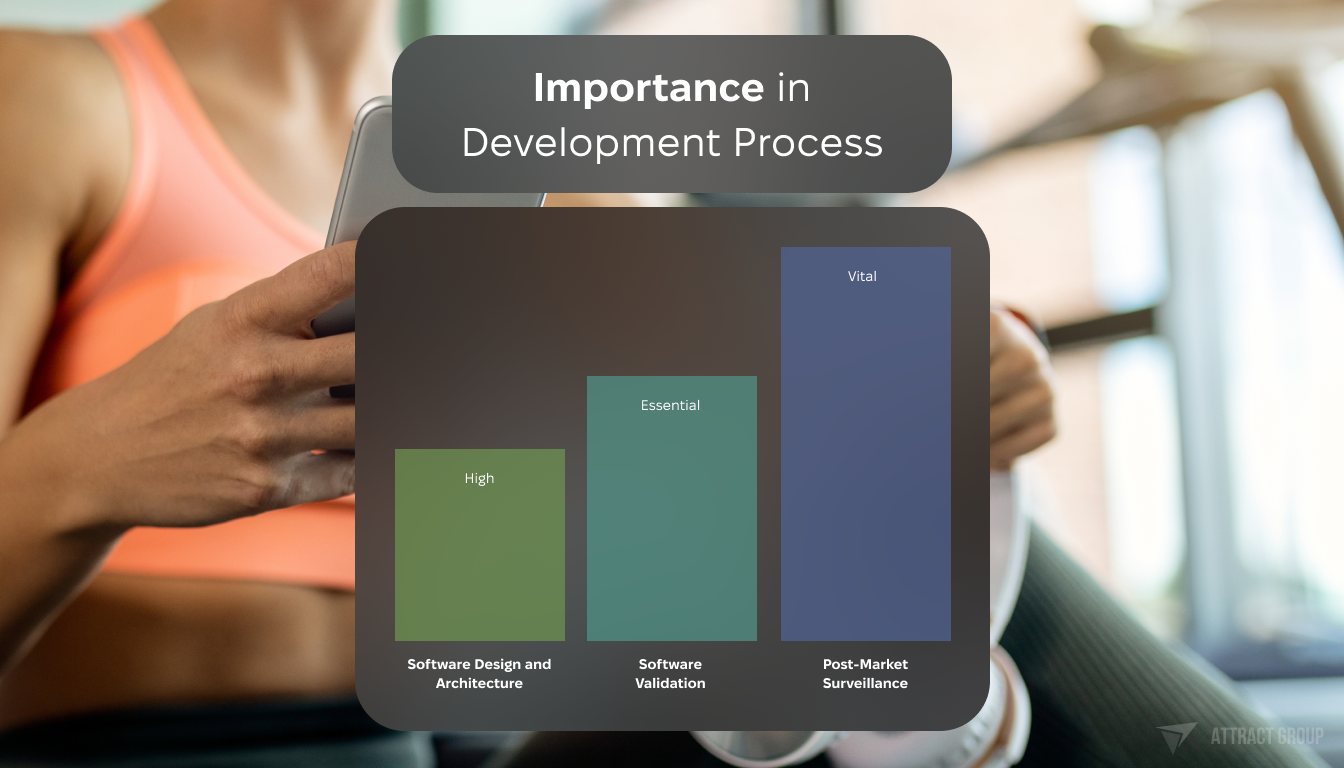
After launching the software, it’s important to keep an eye on it. Watching how the software performs out in the world helps quickly fix any problems. This keeps the software reliable and safe over time.
| Focus Area | Compliance Requirement | Importance in Development Process |
|---|---|---|
| Software Design and Architecture | Conformity with IEC 62304 | High – forms baseline of functionality and interoperability |
| Software Validation | Verification against FDA standards and ISO 13485 | Essential – ensures reliability and safety of software |
| Post-Market Surveillance | Ongoing assessment as per FDA and IEC standards | Vital – monitors real-world performance and compliance |
Cybersecurity Considerations in Medical Software
As medical technology advances, cybersecurity in medical software has become essential. Protecting data integrity and patient info is critical in Software as a Medical Device (SaMD). With cyber threats growing, adopting cyber nuclear best practices is crucial.
Threat Identification and Management in SaMD
Threat identification is vital for SaMD cybersecurity. Cyber threats include ransomware attacking hospitals and phishing targeting healthcare workers. Recent guidelines boost threat awareness and response, like the FDA’s May 1, 2023, video on healthcare cybersecurity preparedness.
Adopting standards from the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC) improves SaMD risk management. These standards help devices withstand and recover from attacks.
Implementing Cybersecurity Best Practices
Following cybersecurity best practices prevents and responds to threats. This means updating systems, running penetration tests, assessing risks, and using strong encryption. The FDA’s guidance from March 13, 2024, stresses proactive defenses and vulnerability testing in medical devices.
Starting with cybersecurity in early SaMD development stages is key. It involves secure coding to prevent unauthorized access and data breaches. This protects against insider threats and cloud storage misconfigurations, which might expose sensitive data.
To visualize the crucial aspects of cybersecurity in medical software effectively, below is a detailed breakdown:
| Aspect | Practice | Significance |
|---|---|---|
| Threat Management | Regular Risk Assessments | Identifies potential vulnerabilities in the system to prevent exploits. |
| Data Integrity | Robust Encryption Protocols | Ensures that patient data is stored securely, mitigating risks of data breach. |
| Regulatory Compliance | Adherence to FDA Guidelines | Maintains market access and establishes trustworthiness of SaMD products. |
| Proactive Defense | Timely Updates and Patch Management | Mitigates emerging threats and addresses security vulnerabilities swiftly. |
Usability Engineering for Medical Software
The use of usability engineering for medical software, especially Software as a Medical Device (SaMD), is crucial. It ensures patient safety and product efficiency. From 2012 to 2015, user interface (UI) software errors caused 423 medical device recalls. This shows the need for good usability engineering.
Frameworks like IEC 62366, which includes IEC 62366-1 and IEC 62366-2, are backed by the FDA. They help developers make easy-to-use interfaces for people. These guidelines aim to find and lessen the risk of user-related hazards. This makes the software safer and less likely to have errors.
Importance of Usability in SaMD
Usability in SaMD is very important. It has been shaped by psychology, engineering, and physiology since after World War II. Many medical mistakes are due to bad design, not user errors. So, the medical software industry combines usability engineering into their work.
This effort involves software creators, cognitive scientists, and ergonomists working together. Their goal is to make software that’s easy to use and meets legal standards. This focus helps improve how people use the software and boosts patient care.
Integrating Usability Engineering Process
The usability engineering process in medical software includes many steps. It starts with reviewing data and defining user interface requirements. Then, there are formative and summative evaluations at various development stages. These steps make sure the software meets both user needs and regulatory standards.
By looking into possible risks during development and revising designs after evaluations, developers reduce the chance of errors. Following a clear usability engineering plan helps make products safer. It also improves the results for patients and user happiness. This supports meeting legal requirements and getting their products to market.
Conclusion
In conclusion, the landscape of medical device software development is continuously evolving, with ISO standards playing a pivotal role in ensuring quality and safety. Medical device manufacturers must navigate complex regulatory requirements while striving for innovation. The implementation of robust software architecture and rigorous code review processes are essential to prevent hazardous situations and maintain traceability throughout the product lifecycle.
As Software as a Medical Device (SaMD) becomes increasingly prevalent, regulatory bodies worldwide are adapting their guidelines to address the unique challenges posed by these digital health solutions. Adhering to the applicable standards not only facilitates compliance but also enhances the overall quality of the final deliverable.
The journey from concept to market for medical software is intricate, requiring a deep understanding of both technical and regulatory aspects. By embracing these standards and best practices, manufacturers can develop safer, more effective products that ultimately benefit patient care and advance the field of medical technology.










